Word Equation to Describe Electrolysis of a Molten Ionic Compound
I can safely electrolyse a solution with guidance provided. At the positive electrode.

Electrolysis Of Molten Ionic Compounds Study Com
Magnesium chloride must be heated until it is molten before it will conduct electricityElectrolysis separates the molten ionic compound into its elements.
. Mg 2 2e- Mg magnesium metal at the -cathode. The lead II Pb2 is attracted to the negative electrode where it gains electrons. Electrolysis of molten ionic compounds.
Electrolysis of Molten Ionic Compounds Instructor. He introduced the term electrolysis in 1834. What is the word equation for the electrolysis of Aluminium oxide.
For example lead bromide can be used to. ELECTROLYSIS OF IONIC SOLUTIONS. Michael Faraday was a pioneer in the field of electrolysis.
Pb 2 ions gain electrons at the cathode and become Pb atoms Br - ions lose electrons at the. Learning outcomes After completing this activity you should be able to. Extraction of Aluminium - Electrolysis Cell.
They receive electrons and are reduced. Electrolysis of a molten ionic compound. 23 Foundation With support I can define electrolysis.
4OH 4e 2H 2 O O 2. The electrolysis of sodium chloride solution NaClaq. Separates the molten ionic compound into its elements.
Electrode for the electrolysis of a molten ionic compound. Faradays Charge 96000. Hemnath Vikash Seeboo Show bio Taught Science mainly Chemistry Physics and Math at high school level.
This is what happens during electrolysis. Electrolysis of the aluminacryolite solution gives aluminium at the cathode and oxygen at the. I can write a word equation to describe the electrolysis of a molten ionic.
Faradays 6000 96000 00625. 2Cl- - 2e- Cl 2 chlorine gas at the anode. B write the balanced net-ionic equation for the half reaction that occurred at the anode.
Describe electrolysis in terms of movement of ions. The suffix lysis is a Greek word meaning break down. I can explain the classification of I can describe electrolysis with half equations at the electrodes.
The half equation is. When an electric current is applied the positive ions move towards the negative electrode. I can state the products of the electrolysis of brine and a use for each.
The bromide Br is attracted to the positive electrode where it loses electrons. Pb2 2e- Pb. Positively-charged ions move to the negative electrode cathode.
Write a balanced symbol equation including state symbols for the overall electrolysis of a molten ionic compound. I can write a word equation to describe the electrolysis of aluminium oxide. Pb 2 2 e - Pb lead metal at the - cathode.
What happens during the electrolysis of molten ionic compounds. The reactions at each electrode are called half equations. A based on the litmus paper test identify the gas produced at the anode.
D write the balanced. If the negative ion from the ionic compound. 2 Br - - 2 e -.
C62 Changes at the electrodes the anode when some solutions are. Describe electrolysis in terms of the ions present and the reactions at the electrodes. Of electrons occur in each equation.
Positive Anode Negative Cathode. 2 For the electrolysis of molten zinc chloride. Calculate the number of moles of the substance produced according to.
KCl 1 Product at cathode 2 Half equation at cathode 3 Product at anode 4 Half equation at anode. Electrolysis of molten salts New substances form when a molten or dissolved ionic compound conducts electricity. In order for a substance to be an electrolyte it must contain an ionic compound and the ionic compound must either be molten or dissolved in an aqueous solution.
Calculate the number of faradays involved. The steel container is coated with carbon and this is used as the negative electrode. Electrolysis of Magnesium Chloride.
2Br Br 2 2e. Describe the experimental set-up for electrolysis write a word equation to describe electrolysis of a molten ionic compound describe electrolysis in terms of movement of ions. When it is melted the Al 3 and O 2-ions are free to move and conduct electricity.
PbBr 2 l Pbl Br 2 g Remember. 2O 2 - O 2 4e -. C write the balanced net-ionic equation for the half reaction that occurred at the cathode.
Analysing the electrolysis of molten compounds Electrolysis is a process whereby a compound is decomposed into its constituent elements when an electric current passes through an electrolyte. Electrolysis is the process by which ionic substances are decomposed broken down into simpler substances when an electric current is passed through them. Before it will conduct electricity.
I can state that oxygen can be produced at the reactions at each electrode as oxidation or reduction. It shows that oxide ions. Write a word equation to describe the electrolysis of a molten ionic compound.
Solid Lead and Bromine Gas are formed. Charge Current Time Q I T Charge 5 20 60 6000. Oxidation Is the Loss of electrons and Reduction Is the Gain of electrons.
However the electrolysis of sodium chloride solution produces hydrogen. Aluminium oxide Al 2 O 3 is an ionic compound. The half equations are.
During electrolysis of molten leadII bromide PbBr 2 l.
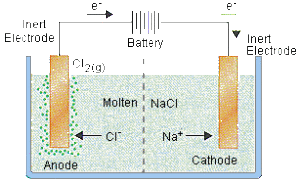
Electrolysis Of Molten Compounds Mini Chemistry Learn Chemistry Online
![]()
Electrolysis Of Molten Ionic Compounds Study Com
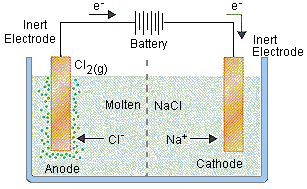
Electrolysis Of Molten Compounds Mini Chemistry Learn Chemistry Online
No comments for "Word Equation to Describe Electrolysis of a Molten Ionic Compound"
Post a Comment